NO ASSOCIATION OF α -ACTININ-3 (ACTN3) AND VITAMIN D RECEPTOR (VDR) GENOTYPES WITH SKELETAL MUSCLE PHENOTYPES IN YOUNG WOMEN
James P. Gavin & Alun G. Williams
Institutefor Performance Research, Manchester Metropolitan University, Alsager, ST7 2HL, United Kingdom
Abstract
This study investigated association between polymorphisms of α-actinin-3 (ACTN3) and vitamin D receptor (VDR) genes, and the skeletal muscle phenotypes; sprint performance, jump capacity, and knee extensor and flexor strength. Sixty-two non-resistance trained Caucasian females (mean ± SD; 21 ± 4 years) completed 15 m sprint, standing vertical jump, knee extensor and flexor isometric maximal voluntary contraction (MVC) tests. 15 m sprint and vertical jump were assessed using infrared timing gates and a piezoelectric force platform respectively, with knee extensor and flexor strength assessed using isokinetic dynamometry. ACTN3
R577X and VDR BsmI polymorphisms were determined using real-time polymerase chain reaction (PCR). A one-way analysis of variance (ANOVA) was used to examine differences between skeletal muscle phenotypes for the ACTN3 and VDR genotypes. 15 m
sprint(ACTN3: RR = 2.87 ± 0.17 s, RX = 2.92 ± 0.22 s, XX = 2.95 ± 0.17 s, P = 0.384; VDR: bb = 2.86 ± 0.14 s, Bb = 2.96 ± 0.23 s, BB = 2.85 ± 0.21 s, P = 0.194) and standing vertical jump performance (ACTN3 P = 0.112; VDR P = 0.788) were not associated with ACTN3 or VDR genotypes. Neither was any association found between knee extensor MVC and ACTN3 (P = 0.120) or VDR genotypes (P = 0.978), or between knee flexor MVC and ACTN3 (P = 0.852) or VDR genotypes (P = 0.718). The ACTN3 R577X and VDR BsmI polymorphisms do not appear to substantially influence the function of skeletal muscle in Caucasian females.
Key words:Isokineticdynamometry; polymorphism; MVC; exercise genetics; sprint time
INTRODUCTION
The annual human gene map for performance and health-related phenotypes lists over 200 ‘candidate’ genes and genetic regions associated with human physical performance, exercise, and health (Bray et al., 2009). Two such ‘candidate’ genes are the α-actinin-3 (ACTN3) and vitamin D receptor (VDR) genes.
α-actinins are a family of actin-binding proteins responsible for securing actin filaments to the sarcomeric Z-line (Blanchard et al, 1989). The gene ACTN3 encodes for the α-actinin protein which is found in type II muscle fibres (North & Beggs, 1996) responsible for rapid and forceful contraction, but not in type I fibres. A genetic variant at amino acid 577 (R577X), causes arginine (R) to be replaced by a premature stop codon (X). Consequently, XX homozygotes for the R allele have the normally functioning gene. X homozygotes do not produce α-actinin-3 protein in skeletal muscle (North et al., 1999). Since 2003, ACTN3 has received attention regarding human-performance, in particular the R577X polymorphism (Yang et al., 2003; MacArthur & North, 2004; Moran et al., 2007). Investigations have found associations between α-actinin-3 deficiency and elite endurance performance (Lucia et al., 2006; Niemi & Majamaa, 2005), whilst the R allele has been link to sprint (Yang et al., 2003; Niemi & Majamaa, 2005) and power performance (Druzhevskaya et al., 2008). More frequent associations observed when studying females may suggest a gender-dependent role for ACTN3 (Clarkson et al., 2005; Delmonico et al., 2007). However, it is not clear whether the ACTN3 R577X polymorphism has an association with physical performance parameters that are observable in the general population (Ostrander, Huson & Ostrander, 2009).
Vitamin D regulates extracellular calcium (Ca2+) homeostasis in skeletal muscle (Walters, 1992) and is involved in the muscle contractile process (Boland, 1986). Effects of vitamin D are expressed via genomic and non-genomic mechanisms through a vitamin D receptor (VDR) (Norman et al., 1992). Genomic mechanisms occur via the binding of vitamin D to VDR, eliciting gene transcription and subsequently protein synthesis. Non-genomic mechanisms are exerted by the activation of VDR to initiate signal transduction pathways that rapidly open Ca2+ channels.
Found on the VDR gene, the BsmI polymorphism is where an A/G alteration changes the site where translation initiates. The common variant is the G nucleotide (or b allele), whereas the A nucleotide (B allele) has the initiation site downstream. b homozygotes express presence of a restriction site, an element where restriction enzymes act to sever the coding sequence, whereas, B allele carriers have absence of the restriction site. The b allele carriers have a shorter form of VDR gene, which may elicit greater transcriptional activity (Uitterlinden et al., 2002). Elderly women with the bb allele were found to have greater isometric quadriceps and hand grip strength compared to those with the BB genotype (Geusens et al., 1997). Conversely, using premenopausal women Grundberg and colleagues (2004) observed that B homozygotes had greater hamstring strength than b homozygotes. Such contradictory findings may be partly explained by varying methodologies.
The objective of this investigation was to examine association between the ACTN3 R577X and VDR BsmI polymorphisms, and skeletal muscle phenotypes related to strength and power performance in young healthy Caucasian women.
METHODS
Participants
Sixty-two UK Caucasian females aged 18 - 39 years volunteered for the study. Participants confirmed that they were free from neuromuscular disorders likely to affect muscle phenotypes via direct questioning. A questionnaire (Baecke et al., 1982) was used to ensure that only those untrained, with low- to moderate-physical activity levels were selected for testing.
Protocol
Within a two-week period participants visited the laboratory on three occasions. Visit one was for sprint and jump data, visit two was for isokinetic dynamometer (Cybex Norm, Cybex International Inc., NY, USA) familiarisation and blood sample, and visit three was for knee extensor and flexor strength. All visits to the laboratory were separated by at least 48 hours, with volunteers requested to refrain from exercise for the 24 hours prior to testing. Body mass was measured using balance scales (Seca Ltd, Birmingham, UK) and stature with a wall-mounted stadiometer (Holtain Ltd, Crymych, UK).
Prior to all measurements of phenotype, participants completed a 5 minute warm-up at an intensity of ~70% age predicted heart rate maximum on a Monark 834E cycle ergometer (Monark Exercise Ab, Varberg, Sweden) and several practice trials for the forthcoming test. During all phenotype measurements (described in the following paragraphs), if observed values did not plateau by the time the minimum number of trials was completed, further attempts were permitted until a plateau was observed.
Skeletal Muscle Phenotypes
15 m Sprint
Five 15 m sprints with 30 s rest periods were performed. Sprint time was determined by the duration to disrupt the first, and then second infrared beam on the timing gates (Department of Exercise and Sport Science, MMU Cheshire, Alsager, UK) placed 15 m apart. Participants were directed to the start line and instructed to commence the sprint on their volition, with instruction not to decelerate until passing the second gate.
Standing Vertical Jump
Vertical jump height was tested with five trials separated by 30 s rest intervals. From the squat position, technique was monitored and verbal instruction was provided by the investigator. Jump height was measured using a piezoelectric force platform (Kistler Instruments Ltd, Hampshire, UK) and calculated from flight time as described elsewhere (Moir, 2008).
Knee Extensor and Flexor Isometric MVC
Using isokinetic dynamometry, maximal knee extensor and flexor torque of the right leg were determined during assessments of isometric MVC. Seated upright, the knee joint angle was fixed at 90° with the right lateral femoral epicondyle aligned to the rotational axis of the isokinetic dynamometer. An ankle pad lever arm was secured to the ankle joint.
Five isokinetic extension and flexion movements (60°·s-1) through a full knee joint range of motion, followed by one submaximal isometric contraction, were used as specific preparation. To test strength, participants performed three MVCs, with 60 s intervals between repetitions. Contraction was maintained for ~3 s, with verbal encouragement and visual feedback provided. The highest MVC value of the three trials was recorded as maximal isometric strength. The protocol was then repeated for knee flexors.
Blood Collection and Genotyping
DNA was extracted from a 0.5 mL whole blood sample obtained via a finger prick. Blood was stored in EDTA-coated tubes at -20°C, DNA was then isolated using a QIAamp DNA Blood Mini Kit and Qiacube automated device (Qiagen, Crawley, West Sussex, UK).
Genotypes were determined using polymerase chain reaction (PCR) in real-time (Chromo4, Bio-Rad Laboratories, Ltd., Hertfordshire, UK). Genotyping assay mixes that include pre-designed primers and probes for rs1815739 (ACTN3) and rs1544410 (VDR), and Taqman® Genotyping Master Mix, were acquired from Applied Biosystems (Foster City, CA, USA). The reactions were all of 10 µL volume, using an initial 10 min at 95°C, then 40 cycles of 15 s at 92°C and 60 s at 60°C. Genotypes were determined using Opticon Monitor 3.1 software linked to the Chromo 4 thermal cycler. All analyses were duplicated and agreement between duplicates was 100%.
Statistical Analysis
To confirm Hardy-Weinberg equilibrium a Pearson’s Chi-square test was applied. A one-way ANOVA was used to examine potential differences in skeletal muscle phenotypes between genotype groups. All statistical analyses were conducted using SPSS 16.0 for Windows. A P value of ≤0.05 was accepted as statistically significant.
RESULTS
Participant Characteristics
The physical characteristics of the sixty-two Caucasian female participants were: age 21.6 ± 4.3 years, height 165.7 ± 5.5 cm, body mass 65.5 ± 10.6 kg, BMI 23.8 ± 3.6 kg·m-2. The genotype distributions conformed to Hardy-Weinberg equilibrium (ACTN3: RR 22, RX 29, XX 11; VDR: bb 22, Bb 30, BB 10). All physical characteristics were independent of genotype.
15 m Sprint
No significant differences were observed between ACTN3 (P = 0.384) or VDR (P = 0.194) genotypes for 15 m sprint time (Figure 1).
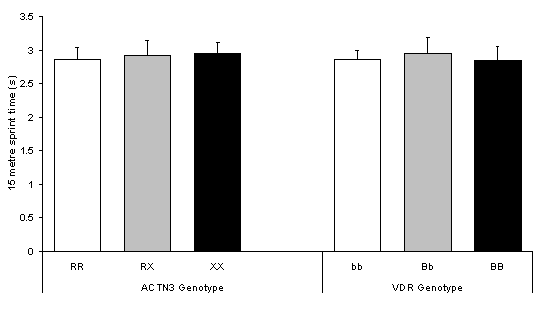
Figure 1. No difference in 15 m sprint time between ACTN3 genotypes (P = 0.384) and VDR genotypes (P = 0.194). Values are mean ± SD.
Standing Vertical Jump
ACTN3 X homozygotes tended to produce greater jump height (0.50 ± 0.04 m) in comparison to R homozygotes (0.44 ± 0.10 m) and heterozygotes, but the differences were not statistically significant (P = 0.112). Neither was jump height significantly different between VDR genotypes (P = 0.788) (Figure 2).
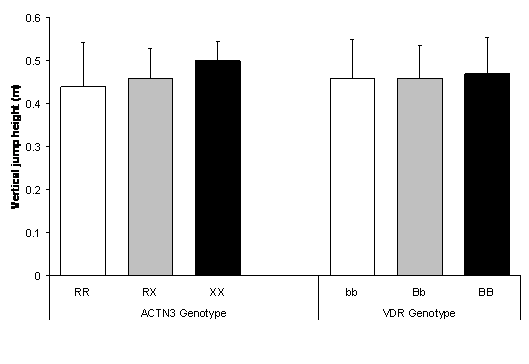
Figure 2. No difference in vertical jump height between ACTN3 genotypes (P = 0.112) and VDR genotypes (P = 0.788). Values are mean ± SD.
Knee extensor and flexor isometric MVC
Knee extensor MVC showed no significant difference (P = 0.120) between ACTN3 genotypes (Figure 3). Neither was MVC significantly different (P = 0.978) between VDR genotypes.
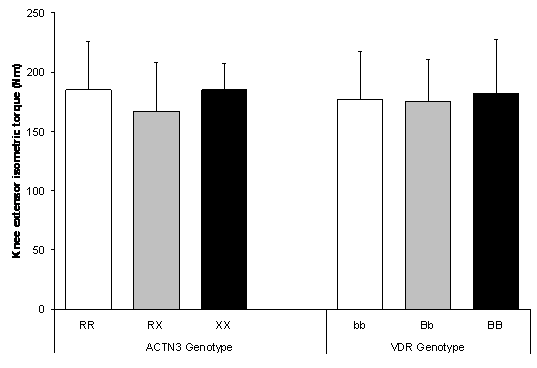
Figure 3. No difference in isometric knee extensor MVC between ACTN3 genotypes (P = 0.120) and VDR genotypes (P = 0.978). Values are mean ± SD.
Knee flexor isometric MVC was similar between all ACTN3 and VDR genotypes (Figure 4). No significant difference existed between ACTN3 genotypes (P = 0.852) or VDR genotypes (P = 0.718).
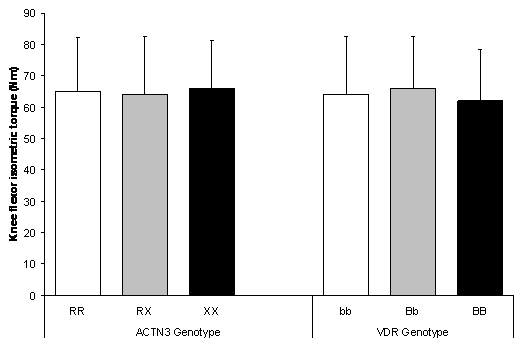
Figure 4. No difference in isometric knee flexor MVC between ACTN3 genotypes (P = 0.852) and VDR genotypes (P = 0.718). Values are mean ± SD.
This investigation examined the association of ACTN3 R577X and VDR BsmI polymorphisms with skeletal muscle phenotypes in women. Our results suggest that in Caucasian females, neither complete deficiency of α-actinin-3 protein, nor the promotion of VDR transcriptional activity, are associated with maximal muscle strength and power. Both polymorphisms have the potential to be associated with human performance phenotypes as evidenced by some of the earlier reports, but the extent of that association does not appear to be sufficient to notably influence the parameters we assessed in young, healthy Caucasian females.
No association was found between sprint or vertical jump performance and genotype in the current study. In Greek adolescents, Moran et al. (2007) found that R homozygotes had faster 40 m sprint times when compared to X homozygotes, although even in that study ACTN3 R577X genotype accounted for just 2.3% of the variability in sprint time. Moran et al. also observed no association with other functional phenotypes such as vertical jump and agility run. Whether there is some element of the physiological determinants of 40 m sprinting that is influenced by ACTN3 genotype, but which is not an important determinant of the 15 m sprint and vertical jump tests we used, such as the ability to move a limb at high velocity or the ability to produce sufficient impulse in the short period of time the foot is in contact with the floor during faster running velocities, remains to be determined. Another recent investigation found no association between genotypes of ACTN3 and the angiotensin-converting enzyme (ACE) gene and muscle function in young males (McCauley et al., 2009). Both isometric and isokinetic tests of voluntary muscle function were used by McCauley et al., as well as a number of parameters in response to maximal twitches induced by percutaneous electrical stimulation. As observed in the current study, McCauley et al. reported no significant associations with ACTN3 genotype. Thus, despite the fact that ACTN3 genotypes have been associated with elite physical performance on several occasions (Druzhevskaya et al., 2008; Lucia et al., 2006; Niemi and Majamaa, 2005; Yang et al., 2003), the practical significance of α-actinin-3 deficiency in non-athletes appears to be minor or non-existent, given the data presented in the current study and some previous reports (Delmonico et al., 2007; San Juan et al., 2006; Moran et al., 2007; McCauley et al., 2009).
In one of the earliest studies of its kind, Geusens et al. (1997) reported that VDR genotype was associated with two different measures of muscle isometric strength in women, finding greater strength associated with the presence of the b allele. In contrast, a later study by Roth et al. (2004) reported no association between the same VDR polymorphism and quadriceps isometric strength in older men. Our results from young females add weight to the hypothesis that there is no influence of VDR BsmI genotype on muscle strength. While serum vitamin D has recently been demonstrated to be positively associated with jump height in adolescent girls (Ward et al., 2009), we nevertheless observed no association of VDR genotype with functional aspects of physical performance related to muscle power, namely sprint and vertical jump abilities.
Human performance phenotypes are mediated by a range of extrinsic and intrinsic factors, of which arguably the most important are training and environmental stress (extrinsic), and genetic constitution (intrinsic) (Rupert, 2003). Skeletal muscle can undergo altered structure and function as a consequence of environmental stresses, resulting in altered gene expression. Cellular signaling and messenger pathways translate mechanical stress imposed on the active muscle tissue into specific adaptation (Garcia-Roves et al., 2006). It may be that the ACTN3 and VDR polymorphisms studied here have an influence on skeletal muscle function that only becomes identifiable in elite populations where the extrinsic factors of training and environmental stress are perhaps relatively homogenous, and thus genetic predisposition may be permitted to play a greater role in differentiating between levels of competitive performance.
Single genetic polymorphisms may typically be insufficient to elicit notable inter-individual differences in physical function (Williams & Folland, 2008). It may be that the overall genetic influence on physical performance traits in non-athletes will only be revealed when polygenic profiling is utilized, although the power of the polygenic profile approach is itself entirely dependent on the evidence used to justify the inclusion of a particular polymorphism in any genetic algorithm used (Williams and Folland, 2008). This paradox describes the challenge facing researchers in this field for the foreseeable future.
CONCLUSION
Results suggest that the ACTN3 R577X and VDR BsmI polymorphisms do not influence skeletal muscle phenotypes in young healthy UK Caucasian females. Such phenotypes are determined by various physiological parameters. Even if an individual possesses a repertoire of favourable genotypes they are unlikely to endow with strength and power performance without environmental contribution. The roles of these particular polymorphisms do not appear to be substantial as to manifest superior strength and power performance in the female cohort investigated. Future research will need to examine much larger homogenous cohorts, with particular consideration to the specificity of performance tests to proposed gene action.
LITERATURE
-
Baecke, J.A., Burema, J., & Frijters, J. R. (1982). A short questionnaire for the measurement of habitual physical activity in epidemiological studies. American Journal of Clinical Nutrition, 36, 936-942.
-
Blanchard, A., Ohanian, V., & Critchley, D. (1989). The structure and function of α-actinin-3. Journal of Muscle Research and Cell Motility, 10, 280-289.
-
Boland, R. (1986). Role of vitamin D in skeletal muscle function. Endocrine Reviews, 7, 434-448.
-
Bray, M.S., Hagberg, J.M., Pérusse, L., Rankinen, T., Roth, S.M., Wolfarth, B., et al. (2009). The human gene map for performance and health-related fitness phenotypes: the 2006-2007 update. Medicine & Science in Sports & Exercise, 41, 34-72.
-
Clarkson, P.M., Devaney, J.M., Gordish-Dressman, H., Thompson, P.D., Hubal, M.J., Urso, M., et al. (2005). ACTN3 genotype is associated with increases in muscle strength in response to resistance training in women. Journal of Applied Physiology, 99, 154-163.
-
Delmonico, M.J., Kostek, M.C., Doldo, N.A., Hand, B.D., Walsh, S., Conway, J.M., et al. (2007). Alpha-actinin-3 (ACTN3) R577X polymorphism influences knee extensor peak power response to strength training in older men and women. Journal of Gerontology: Series A Biological Sciences and Medical Sciences, 62, 206-212.
-
Druzhevskaya, A.M., Ahmetov, I.I., Astratenkova, I.V., & Rogozkin, V.A. (2008). Association of the ACTN3 R577X polymorphism with power athlete status in Russians. European Journal of Applied Physiology, 103, 631-634.
-
Garcia-Roves, P.M., Huss, J., & Holloszy, J.O. (2006). Role of Calcineurin in exercise-induced mitochondrial biogenesis. American Journal of Physiology: Endocrinology and Metabolism, 290, 1172-1179.
-
Geusens, P., Vandevyver, C., Vanhoof, J., Cassiman, J.J., Boomen, S., & Raus, J. (1997). Quadriceps and grip strength are related to vitamin D receptor genotype in elderly nonobese women. Journal of Bone and Mineral Research, 12, 2082-2088.
-
Grundberg, E., Brandstrom, H., Ribom, E.L., Ljunggren, Ö., Mallmin, H., & Kindmark, A. (2004). Genetic variation in the human vitamin D receptor is associated with muscle strength, fat mass and body weight in Swedish women. European Journal of Endocrinology, 150, 323-328.
-
Lucia, A., Gomez-Gallego, F., Santiago, C., Bandres, F., Earnest, C., Rabadan, M., et al. (2006). ACTN3 genotype in professional endurance cyclists. International Journal of Sports Medicine, 27, 880-884.
-
McCauley, T., Mastana, S.S., Hossack, J., MacDonald, M., & Folland, J.P. (2009). Human angiotensin-converting enzyme I/D and α-actinin-3 R577X genotypes and muscle functional and contractile properties. Experimental Physiology, 94, 81-89.
-
MacArthur, D.G., & North, K.N. (2004). A gene for speed? The evolution and function of α-actinin-3. Bioessays, 26, 786-795.
-
Moir, G.L. (2008). Three different methods of calculating vertical jump height from force platform data in men and women. Measurement in Physical Education and Exercise Science, 12, 207-218.
-
Moran, C.N., Yang, N., Bailey, M.E., Tsiokanos, A., Jamaurtas, A., MacArthur, D.G., et al (2007). Association analysis of the ACTN3 R577X polymorphism and complex quantitative body composition and performance phenotype in adolescent Greeks. European Journal of Human Genetics, 15, 88-93.
-
Niemi, A.K., & Majamaa, K. (2005). Mitochondrial DNA and ACTN3 genotypes in Finnish elite endurance and sprint athletes. European Journal of Human Genetics, 13, 965-969.
-
Norman, A.W., Nemere, I., Zhou, L.X., Bishop, J.E., Lowe, K.E., Maiyar, A.C., et al. (1992). 1,25 (OH)2-vitamin D3, a steroid hormone that produces biologic effects via both genomic and nongenomic pathways. Journal of Steroid Biochemistry and Molecular Biology, 41, 231-240.
-
North, K. N., & Beggs, A. H. (1996). Deficiency of a muscle isoform of α-actinin (α-actinin-3) in merosin-positive congenital muscular dystrophy. Neuromuscular Disorders, 6, 229-235.
-
North, K.N., Yang, N., Wattanasirichaigoon, D., Mills, M., Easteal, S., & Beggs, A.H. (1999). A common nonsense mutation results in α-actinin-3 deficiency in the general population. Nature Genetics, 21, 353-354.
-
Ostrander, E., Huson, H., & Ostrander, G (2009). Genetics of athletic performance. The Annual Review of Genomics and Human Genetics, 10, 407-429.
-
Roth, S.M., Zmuda, J.M., Cauley, J.A., Shea, P.R., & Ferrell, R.E. (2004). Vitamin D receptor genotype is associated with fat-free mass and sarcopenia in elderly men. Journal of Gerontology, 59, 10-15.
-
Rupert, J.L. (2003). The search for genotypes that underlie human performance phenotypes. Comparative Biochemistry and Physiology Part A, 136, 191-203.
-
San Juan, A.F., Gomez-Gallego, F., Cañete, S., Santiago, C., Pérez, M., & Lucia, A. (2006). Does complete deficiency of muscle alpha actinin 3 alter functional capacity in elderly women? A preliminary report. British Journal of Sports Medicine, 40, 1-3.
-
Uitterlinden, A.G., Fang, Y., Bergink, A.P., van Meurs, J., van Leeuwen, H., & Pols, H. (2002). The role of vitamin D receptor gene polymorphisms in bone biology. Molecular and Cellular Endocrinology, 197, 15-21.
-
Walters, M.R. (1992). Newly identified actions of the vitamin D endocrine system. Endocrine Reviews, 18, 719-764.
-
Ward, K., Das, G., Berry, J., Roberts, S., Rawer, R., Adams, J., et al (2009). Vitamin status and muscle function in post-menarchal adolescent girls. Journal of Clinical Endocrinology & Metabolism, 94, 559-563.
-
Williams, A.G., & Folland, J.P. (2008). Similarity of polygenic profiles limits the potential for elite human physical performance. Journal of Physiology, 586, 113-121.
-
Yang, N., MacArthur, D.G., Gulbin, J.P., Hahn, A.G., Beggs, A.H., Easteal, S., et al. (2003). ACTN3 is associated with human elite athletic performance. The American Journal of Human Genetics, 73, 627-631.
NE POSTOJI VEZA IZMEĐU α-AKTININA-3 (ACTN3) I GENOTIPA RECEPTORA VITAMINA D (VDR) SA SKELETNOMIŠIĆNIM FENOTIPOM KOD DJEVOJAKA
Sažetak
Istraživanjem se tražila veza između polimorfizma α-actinina-3 (ACTN3) i gena receptora vitamina D (VDR) sa jedne strane i skeletnomišičnog fenotipa; brzine trčanja, skakačkog kapaciteta i snage fleksora i ekstenzora koljena. Šezdeset dvije djevojke, bjelkinje (mean ± SD; 21 ± 4 godine) koje nisu bile uključene u bilo kakav trening snage su izvele testove: sprint 15m, vertikalni skok iz mjesta i maksimalnu voljnu kontrakciju ekstenzora i fleksora koljena (MVC). Test 15m sprint je izveden koristeći se infracrvenim vremenskim kapijama, skok u vis iz mjesta izveden na tenziometrijskoj platform, a MVC test je izveden na izokinetičkom dinamometru. ACTN3 R577X i VDR BsmI su određeni korištenjem polimeričnom lančanom reakcijom (PCR) u stvarnom vremenu. Analiza varijanse (ANOVA) je korištena kako bi se utvrdile razlike između skeletnomišičnog fenotipa za ACTN3 i VDR genotipove. 15m sprint (ACTN3: RR = 2.87 ± 0.17 s, RX = 2.92 ± 0.22 s, XX = 2.95 ± 0.17 s, P = 0.384; VDR: bb = 2.86 ± 0.14 s, Bb = 2.96 ± 0.23 s, BB = 2.85 ± 0.21 s, P = 0.194) i vertikalni skok iz mjesta (ACTN3 P = 0.112; VDR P = 0.788) nisu u vezi sa ACTN3 i VDR genotipovima. Također, nije pronađena nikakva veza između MVC ekstenzora koljena i ACTN3 (P = 0,120) ili VDR genotipa (P = 0,978) ili između MVC fleksora koljena i ACTN3 (P = 0,852) ili VDR genotipa (P = 0,718). ACTN3 R577X i VDR BsmI polimorfisam značajno ne utiču na funkciju skeletnih mišića kod djevojaka bjelkinja.
Ključne riječi: izokinetička dinamometrija, polimorfizam, maksimalna voljna kontrakcija, genetika vježbanja, vrijeme sprinta
Corresponding author:
Mr. J.P. Gavin, MSc
Institute for Performance Research,
Manchester Metropolitan University,
Hassall Road, Alsager, ST7 2HL, UK